Ciencia & Futuro
V. 13. No. 3 septiembre-noviembre 2023
ISSN 2306-823X
Recibido: 13 mayo 2023/ Aceptado: 18 julio 2023
Determination of Aflatoxins in Maize Grains (Zea Mays L.) Intended for Human Consumption Marketed in Mozambique
Determinación de aflatoxinas en granos de maíz (Zea Mays L.) destinados al consumo humano comercializados en Mozambique
Esnaider Rodríguez Suárez
erodriguezsuarez2013@gmail.com
Amos Nhaca Nhaca
Eduardo Mondlane University (Mozambique)
Abstract: This work aimed to determine aflatoxins in corn grains sold in Mozambique, using the thin layer chromatography (CCD) technique, as well as to evaluate their physical quality and safety. The evaluation of the physical quality and safety of the maize grains consisted of counting insects (live and dead), determining grains that were attacked, broken, defective and impurities through entomological tests and the moisture of the grains was determined through the humidity kit. In the analyzed samples, it was verified the absence of aflatoxins in one sample and the presence of only group B aflatoxins in the remaining ones, whose levels vary from 1,67 to 8,33μg/Kg. Aflatoxin contamination levels are below the limit allowed by national legislation (20μg/Kg). The moisture content in the samples varied between 13, 3% and 16, 5%, which means that some samples have low values and above 14%, the maximum value acceptable. While evaluating the physical quality and safety of the grains, it was found that the samples have a strong infestation by insects of different types per kg of sample, with Sitophilus Zeamais being the species that most attacks and causes damage to the stored grains. The analyzed samples showed a high percentage of attacked grains (which varies between 4 and 10,2%), broken grains between 5,0 and 6,0%, impurities between 4,2 and 5,5%, where the values were far above the maximum allowed by the same standard whose maximum limits are 3%, 3% and 0,1%, respectively.
Keywords: cereal; chromatography technique; grass; human diet; mycotoxina
Este trabajo tuvo como objetivo determinar aflatoxinas en granos de maíz comercializados en Mozambique, mediante la técnica de cromatografía en capa fina (CCD), así como evaluar su calidad física e inocuidad. La evaluación de la calidad física e inocuidad de los granos de maíz consistió en el conteo de insectos (vivos y muertos), determinación de granos atacados, quebrados, defectuosos e impurezas mediante pruebas entomológicas y se determinó la humedad de los granos mediante el kit de humedad. En las muestras analizadas se verificó la ausencia de aflatoxinas en una muestra y la presencia de aflatoxinas únicamente del grupo B en las restantes, cuyos niveles varían de 1,67 a 8,33μg/Kg. Los niveles de contaminación por aflatoxinas están por debajo del límite permitido por la legislación nacional (20μg/Kg). El contenido de humedad en las muestras varió entre 13,3% y 16,5%, lo que hace que algunas muestras presenten valores bajos y superiores al 14%, el valor máximo aceptable. Al evaluar la calidad física e inocuidad de los granos, se encontró que las muestras presentan una fuerte infestación de insectos de diferentes tipos por kg de muestra, siendo Sitophilus Zeamais la especie que más ataca y causa daños a los granos almacenados. Las muestras analizadas mostraron un alto porcentaje de granos atacados (que varía entre 4 y 10,2%), granos quebrados entre 5,0 y 6,0%, impurezas entre 4,2 y 5,5%, donde los valores estuvieron muy por encima del máximo permitido por la misma norma cuyos límites máximos son 3%, 3% y 0,1, respectivamente.
Palabras clave: técnica de cromatografía; gramínea; cereal; dieta; micotoxina.
Introduction
The diet of the population is traditionally based on the consumption of cereals such as corn, rice, wheat, which are considered very energetic foods, as they contain vitamins, proteins,carbohydrates, lipids and minerals that are essential for the human body. Corn, scientifically known as Zea mays L., is widely and commonly consumed in nature, as green and in the form
of by-products such as flour and dough, when dried (Aquino, 2003). Corn stands out in relation to other cereals for its greater diversity of applications and as an excellent source of raw material for more than 500 food products (Castro, 2009). Maize is one of the most cultivated and ancient crops known, occupying the second place in production after wheat, with rice in third place, produced in varied climates from temperate to tropical zones (Augusto, 2016; Heck, et al., 2020; Ortega, 2023).
In Mozambique, maize crops cover a total area of about 29 %, the family sector being the most important in its production and it is considered an income and subsistence crop (Mudema, 2012). Corn is consumed in different ways, such as roasted, cooked (fresh corn) and in the form of flour (dried corn) and is an essential raw material for the preparation of local alcoholic beverages. In addition to direct food consumption, maize is used by industries producing other products based on maize flour, such as poultry feed (POCC, 2018).
Therefore, its quality is one of the most important requirements for its consumption, and is directly related to the purity of the grains, amount of broken and attacked grains, impurities, moisture content, presence of fungi and mycotoxins. Corn grains can be damaged by fungi during pre- and post-harvest, whose damage consists of mold, discoloration and unpleasant smell, chemical changes and reduction of nutritional quality, representing a risk to food safety, as they become if unfit for human and animal consumption (Lima, 2017).
The presence of some species of fungus in grains can lead to the production and accumulation of secondary metabolites that are toxic to humans and animals, called mycotoxins, and can cause diseases (mycotoxicosis) (Lima, 2017). The consumption of food contaminated by mycotoxins is a public health issue worldwide and is associated with great damage to the economy and agribusiness in many countries. The World Organization for Agriculture and Food (FAO), states that many products are lost in the world annually due to fungi and mycotoxins and that 40% of the reduction in life expectancy in poor countries is related to the existence of mycotoxins in the diet of their populations.
Aflatoxins are a group of mycotoxins produced by strains of the fungal genus Aspergillus, with A. flavus and A. parasiticus being the most important species, and when ingested through food products, they can lead to the development of various pathologies in humans, such as liver cancer. In view of this situation, corn grains, being one of the agricultural products most affected by fungi of the genus Aspergillus and consequently producing aflatoxins, the present work aims to verify the occurrence of aflatoxins in corn grains sold in the country.Mozambique is a potentially agricultural country and has favorable conditions from the point of view of climate and temperature for the growth of toxigenic fungi, a situation aggravated by inadequate agricultural practices and inadequate storage of agricultural products. The development of toxicogenic fungi can occur at all stages of the maize production chain, originating mycotoxins and having a cumulative character in the body when ingested, directly or indirectly, they can cause mild to severe damage, the effects of which vary with the frequency of ingestion, quantity or concentration and toxicity of ingested mycotoxin.Inadequate storage and handling of cereals (grains) lead to loss of quality, because, for example, corn grains are an ecologically living system, although in a state of dormancy, they continue with active functions, such as breathing and perspiration, being prone to physical-chemical alterations by influences of physical, chemical and biological factors. Therefore, the drying method, temperature and storage environment and humidity of the grains are crucial to accelerate or delay the deterioration process, as they can intensify the respiration of the grains, thus facilitating insect infestation and the proliferation of toxigenic fungi.The problem of aflatoxins arises from the agricultural fields, transport, and storage and at the point of sale, due to non-compliance with the measures for the prevention and control of mycotoxins stipulated by FAO through HACCP (The Hazard Analysis Critical Control Point). So far, there is no way of production or storage that guarantees the consumer that there are no mycotoxins. Some thermal treatments and industrial processes can eliminate the fungi present, but they are not capable of eliminating toxins from food.The objective of this study was to determination the aflatoxin content in maize grains for human consumption marketed in Mozambique.
Materials and method
Study area and design
The experimental part of this work had two components: the field component and the laboratory component. The field component was based on the harvest of maize grains in one of the largest wholesale markets in the city of Maputo, called Mercado do Zimpeto, accompanied by a survey. On the other hand, the laboratory component consisted of carrying out analyzes or laboratory experiments.
The samples were acquired in february 2023, in the largest wholesale market in Zimpeto, located in the city of Maputo. The sampling process first consisted of identifying points of sale of corn grains in bulk, where following the rules stipulated by the Mozambican standard NM 4-2009, Cereals Specifications for corn including methods of analysis and sampling by the National Institute of the Normalization e Quality control. They were purchased randomly in different bags and quantities from the same retail outlet.
Sampling technique and sampling procedure
One of the crucial steps in the qualitative and quantitative determination of mycotoxins is sample preparation and pre-concentration. Aflatoxins, being hydrophobic compounds, are extracted using organic solvents. The sample purification process, the high specificity between the anolyte and the extraction techniques affect the sensitivity of the result. The choice of sample preparation method depends on several factors such as matrix type, the nature of the compound to be analyzed (solubility, polarity), among others (Drumond, 2017).
The health quality of grains is related to the presence of fungi, insects and contamination from any source where broken, attacked grains, impurities and insects are subdivided by visual inspection and subsequent weighing (Copetti, 2009; Bento, 2011). The level of infestation (insects) defines the ability to cause damage to products during storage, because of them serving as food, in addition to causing physical changes, reducing their quality (Paim, 2016).
The process of analyzing the quality and safety of the grains consisted of using a circular sieve with a diameter of 4,75 mm, where the grains (about 1 kg) were placed on it and circular movements were performed for an approximate period of 60 seconds, impurities or foreign matter and broken grains passed through the sieve holes and were subsequently separated and their weight was determined. Then, in a visual and attentive way, the defective grains, attacked grains, altered grains and pieces of grain remaining in the sieve were selected and separated, and then weighed separately.
The next stage consisted of counting live and dead insects, where the live ones were placed in a Petri dish containing 60% ethyl alcohol and the type of each insect species was distinguished by observing its morphological characteristics, through a magnifying glass. The percentage of each grain category was determined using the following formula:
![]()
Where:
M1: mass of each category and Ma- sample massThe moisture content of the grain is defined as the weight loss when it is dried and it is expressed as a percentage of the weight of the original sample. However, at present it is determined through expeditious methods, using moisture meters. The determination of grain moisture is important, as it is one of the main factors for fungal infection and subsequent production of aflatoxins (Gabriel, 2014).Analysis of aflatoxinsProcedures for the identification and confirmation of Aflatoxins
The residue was redissolved with 500µL of methanol, stirred for a few seconds and 1 µL was applied on the chromatographic plate (previously activated in an oven for 30 min), the methanolic extract and the standard solutions of B2, G1, and G2 to 1 µg/ml.
The plate was developed in chloroform-acetone (9:1) mobile phase.
The plate was dried at room temperature and developed under ultraviolet light (λ=366 nm) and contamination was confirmed by spraying with H2SO4 solution (1:3).
Procedures for quantification of aflatoxins
A new plate was prepared and 2µL of the sample and 1 µL of the B2, G1 and G2 standards, prepared at a concentration of 2, 1, 0, 5 and 0.1 µg/mL each, were applied and the plate on chloroform-acetone mobile phase (9:1 v/v).
The plate was dried at room temperature and visualized under ultraviolet light (λ=366 nm) and the fluorescence intensity of the stain of the aflatoxins present in the samples was compared with that of the standard and the respective concentrations were calculated. The concentration of aflatoxins is expressed in µg/kg (ppb), calculated using:
Concentration (µg/kg)
=![]()
Where:
S: volume in µL of fluorescence standard equal to that of the sample,
Y: concentration of mycotoxin standard in µg/mL;
Z: volume in µL of the sample extract stain in which its fluorescence intensity was equal to that of the standard.
W: mass in grams of the sample contained in the final extract;
V: final extract volume in µL.
Result and discussion
Analyzes were carried out in triplicate in the determination of moisture, with the results subject to statistical treatment (Grubb's test) considering a confidence level of 95%. The determination of the other parameters were subject to a unitary analysis and were not subjected to statistical treatment.
Analysis of the quality and safety of corn grainsThe quality and safety of maize grains is associated with the presence or absence of pathogens (fungi, bacteria, viruses) and insects. This analysis also aims to determine the health status of the grains, identifying possible anomalies suffered by them. These parameters can be used to determine the commercial value of aflatoxins (Gabriel, 2014). The results of the analyzed parameters are presented in table 1.Table 1. Results of the analysis of the quality and safety of corn grains
Samples |
Categories (in percentage) |
||||||
Insects |
Grains |
||||||
Alive |
Dead |
Atacked |
Defectuous |
Broken |
Diferent color |
Impurities |
|
I |
7 |
0 |
4,0 |
3,0 |
5,0 |
5,6 |
5,0 |
II |
11 |
5 |
8,0 |
7,0 |
4,0 |
3,6 |
5,5 |
III |
10 |
4 |
9,4 |
8,2 |
4,5 |
7,0 |
4,2 |
IV |
15 |
2 |
10,2 |
10,0 |
6,0 |
3,7 |
5,0 |
With the results shown in Table 1 referring to the analysis of the quality and safety of corn grains, in relation to the insect count, sample IV has a greater number of live insects. Sample I has the lowest number of live insects and, on the other hand, has no dead insects. Insects found in stored grains are classified into primary and secondary insects (Elias, 2017). Primers attack whole grains and can be internal or external primaries, depending on the part of the grain attacked. Internal primaries bore, penetrate and feed on the entire interior of the grain (eg Sitophilus zeamais). External primaries destroy the outside of the grain to feed on the inside (Rhyzopertha dominica). Secondary insects feed on grains broken or damaged by primary insects, as they cannot attack whole
grains and only occur in the grain when its physical integrity is compromised and they multiply quickly causing great damage (eg Tribolium and Cryptoletes genera). Table 2 below lists the different insect species identified in the samples. The Information was
taken from the insect classification manual, provided by the entomology laboratory of the National Water and Food Hygiene Laboratory.Table 2. Presentation of the results of the types of live insects found in the samples
|
Point |
Insects |
Specie |
Characteristics |
|
I |
3 |
Sitophilus grananinius |
Attacks all types of grains |
|
Rhizoperta dominica |
Live in warm climate; Attacks all types of grains |
||
|
Dzizaephilus Surinamensis |
Attacks grains and their derivates |
||
|
II |
2 |
Sitophilus Zeamais |
Attacks all types of grains |
|
Rhizoperta dominica |
Live in warm climate; Attacks all types of grains |
||
|
III |
2 |
Sitophilus Zeamais |
Attacks all types of grains |
|
Tribolium confusum |
Lives in grain derivatives and contaminated grain, attacks corn embryo |
||
|
IV |
2 |
Sitophilus zeamais |
Attacks all types of grains |
|
Cryptoletes turicus |
Lives in the grain, attacks the corn embryo |
According to the results obtained when identifying and classifying live insect species in corn grain samples, it can be said that the insects of Sitophilus Zeamais species are more prevalent in all samples, characterized by attacking all types of grain, mainly the stored grains or during their storage, surviving in the grains through the consumption of the corn borer.
Moisture content in corn kernels
Moisture normally represents the water contained in the sample and its determination is one of the most important measures used in food analysis and is related to its stability, quality and composition (Gabriel, 2014). The table 3 refers to the results obtained in the determination of humidity together with values resulting from the statistical analysis of this parameter, which are the mean, standard deviation, relative standard deviation and confidence limit
Table 3. Presentation of moisture content results
|
|
Humidity |
|||
|
Samples |
||||
|
I |
II |
III |
IV |
|
|
n |
13,5 |
14,3 |
13,6 |
16,6 |
|
13,2 |
14,1 |
13,1 |
16,1 |
|
|
13,3 |
14,6 |
13,5 |
16,4 |
|
|
Media |
13,3 |
14,2 |
13,4 |
16,5 |
|
S |
0,07 |
0,10 |
0,26 |
0,10 |
|
RSD (%) |
0,53 |
0,70 |
1,97 |
0,61 |
|
LC |
13,3±0,6 |
14,2±0,9 |
13,4±0,7 |
16,5±0,9 |
The experimental results presented in Table 3 referring to moisture indicate that the contents varied between 13, 3 and 16, 5%, where sample I with 13, 3 ± 0, 63% and sample IV with 16, 5 ± 0, 90% have lower and higher humidity values, respectively.
Table 4. Statistical analysis values and comparison of calculated G and critical G in determining sample moisture
|
Sampling point |
Parameter |
Valor Suspeito |
x |
S |
n |
Gcalculado |
Gcrítico |
Gcal >Gcrít |
|
I |
Humidity |
13,5 |
13,3 |
0,1291 |
3 |
1,549 |
1,155 |
Yes |
|
II |
14,6 |
14,3 |
0,2082 |
3 |
1,441 |
1,155 |
Yes |
|
|
III |
13,1 |
13,4 |
0,2646 |
3 |
1,133 |
1,155 |
No |
|
|
IV |
16,1 |
16,4 |
0,2082 |
3 |
1,441 |
1,155 |
Yes |
Table 5. Values of t, μ, n and s used in the statistical analysis to calculate the confidence limits
|
Sampling points |
t(n-1) |
s |
μ |
n |
s |
|
I |
12,71 |
0,1528 |
1 |
2 |
0,07 |
|
II |
12,71 |
0,2516 |
1 |
2 |
0,10 |
|
III |
4,30 |
0,2645 |
2 |
3 |
0,26 |
|
IV |
12,71 |
0,2082 |
1 |
2 |
0,10 |
Qualitative identification of aflatoxins in corn kernels
Based on the chromatographic plates shown in Figure 1, the presence of aflatoxins was identified in some samples of corn grains stored and sold in the wholesale market in Zimpeto. Through the same figure, one can observe the fluorescence of the standards (AFB1, AFB2, AFG1 and AFG2) and of the aflatoxins B1 and B2 (Figure 1), demonstrating a possible contamination of the samples.
Figure 1. Chromatographic plates: sprayed with H2SO4 (1:3) (a), illustration of the fluorescence of the standards (B2, G1 and G2) and of the contaminated samples (b).Table 6 below presents the results regarding the detection of different types of aflatoxins in the analyzed samples.
Table 6. Presentation of the results regarding the identification of aflatoxins in the samples. D- Detected; ND- Not Detected
|
Samples |
Aflatoxins |
|||
|
AFB1 |
AFB2 |
AFG1 |
AFG2 |
|
|
I |
ND |
ND |
ND |
ND |
|
II |
ND |
D |
ND |
ND |
|
III |
D |
ND |
ND |
ND |
|
IV |
D |
D |
ND |
ND |
Table 7 shows the values of the aflatoxin content in the contaminated samples.
Table 7. Presentation of the results of the aflatoxin contents in the contaminated samples. NQ- Not Quantified; ND- Not Detected
|
Samples |
Aflatoxins |
||||
|
AFB1 |
AFB2 |
AFG1 |
AFG2 |
Total (μg/Kg) |
|
|
II |
ND |
1,67 |
ND |
ND |
1,67 |
|
III |
NQ |
ND |
ND |
ND |
NQ |
|
IV |
NQ |
8,33 |
ND |
ND |
8,33 |
Table 8. Maximum contents or limits stipulated by national legislation for the different categories of grains
|
Description of specification |
Maximum permissible |
|
Variety grains |
5 % |
|
Unhealthy grains (spoiled) |
0,5 % |
|
Grain attacked by insects |
3 % |
|
Brocken grains |
3 % |
|
Grains with other colors |
2 % |
|
Impurities |
0,1 % |
|
Insects alive |
Absent |
|
Insects dead |
0,1 % |
|
Humidity |
14 % |
|
Micotoxins |
20 ppb |
Discussion
According to table 4, it can be seen that some suspicious values such as "outliers" comply with the null hypothesis condition, that is, with a level of 95% in the Grubb test and were
included in the calculation of the mean, standard deviation and coefficient of variation. Values that did not meet the null hypothesis condition were consequently rejected.
Live and dead insect count
Insect attack causes qualitative and quantitative damage to maize grains and the results of their action translates into weight loss, low germination power, commercial devaluation of the product, dissemination of fungi, etc. The presence of insects in storage alters the physiological functioning and quality of the grain and facilitates the penetration of microorganisms (fungi) (Gabriel, 2014). Figure 2 shows the experimental results of table 1, referring to the counting of live and dead insects in the corn grain samples.
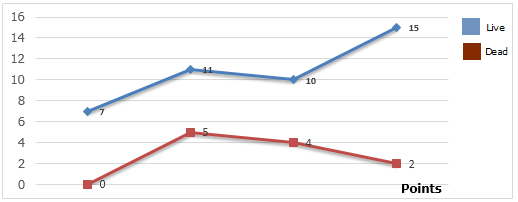
Figure 2. Illustrative on the counting of live and dead insects in corn grain samples.All analyzed samples showed strong infestation by insects of different species, with Sitophilus zeamus being the predominant species, which means that there are no differences in relation
to the sampling site, that is, the insects found in the samples are the same for any sampling point. Sample IV has a higher degree of infestation by live insects, followed by sample III and, on the other hand, sample I does not have any dead insects. A population of insects within the grain mass, if left unchecked can create conditions of increased humidity and temperature thatencourage rapid fungal growth, grain spoilage and mycotoxin production (Pezzini, 2005; Braga, 2017).
A rigorous control of insects is necessary, as in addition to consuming a large part of the mass of the grain, they are vectors for the dissemination of spores, which can initiate or aggravate the proliferation of fungi (Domenico, 2014). In addition, insects, plant diseases, and other biotic and abiotic stresses facilitate infection and aflatoxin production by the fungus (Mohamed, 2017). The moisture of stored grains increases with the increase in the insect population and grains attacked mainly with S. zeamais lose quality during storage because of the increased content of impurities or foreign matter and the incidence of damaged grains (Oliveira, 2015). Unlike fungi, which feed only on the lipid fraction of grains, insects rely on the carbohydrates of the endosperm (starch), their important source of energy.Whole grains
Attacked grains refer to those grains that have holes or that show openings or tunnels, indicating the presence of insects, insect webs or insect waste. Grains or pieces of grain that are attacked by insects considered pests of stored grains in any of their evolutionary stages (NM, 2009). Figure 3 shows the variation in the percentage of grains attacked in corn grain samples.
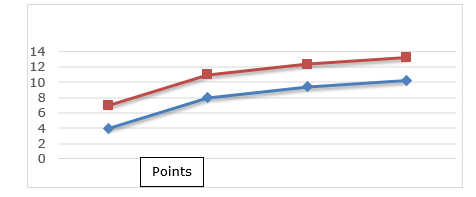
Figure 3. Illustrative of the percentage of grains attacked from corn grain samples.
For this parameter, it was found that all had a percentage greater than the maximum stipulated by NM (2009), which is 3%. The attacked grains increased with the increase of the insect population, as they constitute their food. Damaged grains are more prone to fungal invasion and consequently mycotoxin contamination (Mohamed, 2017).
Defective grains
For this parameter, it was found that all had a percentage greater than the maximum stipulated by NM (2009), which is 3%. The attacked grains increased with the increase of the insect population, as they constitute their food. Damaged grains are more prone to fungal invasion and consequently mycotoxin contamination (Mohamed, 2017).
Defective grainsOne of the main factors that influence the quality of maize grains are the diseases that occur both in the field and in storage, highlighting the pre-harvest rotting of ears, which can result in the formation of defective grains. These grains are usually associated with damage that occurred in the field, post-harvest, during transport and storage (Conceição, 2015). Defective grains are the result of infestation by fungi, both saprophytic (from storage) and phytopathogenic (from the field) (Gabriel, 2014). In the graph below, represented by figure 4, the percentages of defective grains in the maize samples are indicated.

Figure 4. Illustrative percentage of defective grains in corn grain samples.According to figure 3, it can be seen that all samples have high values compared to the maximum stipulated by the Mozambican Standard, which is 0,5%. Defective grains present total or
partial darkening of the endosperm of the grain due to fungal contamination or the action of heat, humidity or fermentation, have mold visible to the naked eye and green or blue color in the germ, produced by the presence of fungi, and have low nutritional value. The increased incidence of defective grains may be related to the delay in harvesting ears, which are vulnerable to attack by field fungi (Conceição, 2015). Contamination with aflatoxins in corn samples was evaluated and it was verified that the fraction that contained grains with defects up to ¼, harmed by different causes and parties, was the main responsible for the level of total contamination of the samples. The separation of the grains with defects could favor a reduction in contamination in corn batches (Bento, 2011).Broken grainsBroken or broken grains are considered to be pieces of grain that pass through the circular sieves of 4,75 mm in diameter, which result from an intense attack by insects or from mechanical damage caused during harvesting and movement of the grain or from thermal damage associated with exposure at high temperatures during drying (Bento, 2011). Broken grains or damaged by insects, favor fungal infection and subsequent production of mycotoxins and states that some types of insects that infest stored grains in which the larval period develops inside the grain due to the presence of broken grains. It brings about a large number of fungal spores from storage become transmitters of these to the rest of the grain mass, gradually reducing its quality (Gabriel, 2014). The figure 5 below indicates the variation in the percentages of broken grains in the samples.
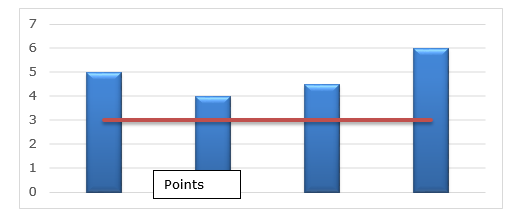
Figure 5. Illustrative percentage of broken grains in corn grain samples.According to the graph above, it is noted that all samples have high values with regard to the maximum stipulated by law, which is 3%. In one study, they observed an increase in the percentage of broken grains as drying temperature and storage time increased. Cracked grains are more susceptible to fungal attack and consequently mycotoxin production than whole grains (Munguambo, 2001).Humidity
The high moisture content in foodstuffs can create an environment conducive to the proliferation of pathogenic microorganisms that can pose risks to the health of the consumer. Humidity is a factor responsible for the deterioration of grains, as it causes an increase in respiration and the number of microorganisms and insects (Gabriel, 2014). Figure 65 shows the average moisture content of corn grain samples from different points in the Zimpeto market.
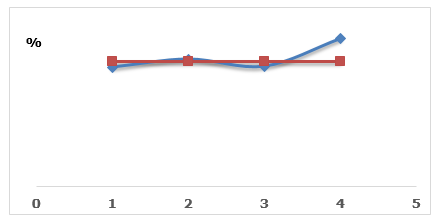
Figure 6. Illustrative of the moisture contents of the samples at different sampling points in the market.
With the determination of moisture content it was found that samples I (13, 3%) and III (13,4%) had slightly low moisture values compared to the values stipulated in national legislation. The remaining samp nsects, as the way in which the corn bags are stored (in the form of large heaps), allows for rewetting of the grains and possible contamination by aflatoxins.Moisture contents below 14% are safe for storage, as they are detrimental to both fungal growth and mycotoxin production (Gabriel, 2014). The ideal humidity for the development of A. flavus in the grain is 18%, however, it also develops in humidity up to 13,1%, as the fungi that produce mycotoxins in grains during storage grow in moisture content of 13- 18% (Lima, 2017). Another researcher recommends that the moisture of corn kernels during storage be 12-13% for a period of 12 months and for longer periods do not exceed 11% (Domenico, 2014). The moisture content is one of the factors that governs the conservation of stored grains; therefore, its monitoring must be done in all stages of production until storage. A moisture content above a safe les II (14, 2%) and IV (16, 5%) show values above the maximum value set in the same standard. Associated with high humidity, we have a higher rate of
defective grains and live i limit favors infestation with fungi and insects, which reduces the shelf life of the product, influencing contamination by mycotoxins (Gabriel, 2014). Studies reveal that high percentages of fungal infestation are related to high moisture content, which leads to a progressive reduction in seed/grain quality (Gabriel, 2014). Therefore, high moisture content may be associated with incomplete drying, storage and sale in places where there are temperature fluctuations, relative humidity, insect proliferation and direct contact with the soil, as these aspects influence the increase in moisture content in the grains.Analysis of groups of identified aflatoxinsFigure 7 shows the levels of quantified aflatoxins (a) and groups of aflatoxins identified in contaminated samples from different points of sale in the Zimpeto market.
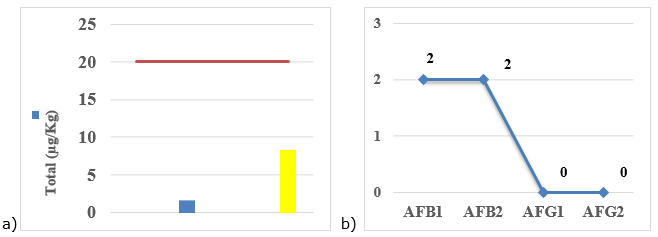
Figure 7. Illustrative of the levels of quantified aflatoxins (a) and groups of aflatoxins identified in samples contaminated with corn grains from different sampling points in the market (b).
After quantification, in particular AFB2, it was found that samples III (1, 67 µg/kg) and IV (8, 33 µg/kg) had low levels in relation to the maximum predicted by the national standard, which is of 20µg/kg (NO. 2009). Despite the low concentration, it is ideal that foods are free of mycotoxins, as frequent and prolonged ingestion of foods with low concentrations of aflatoxins can cause acute poisoning.
Of the aflatoxins found, the groups present in the contaminated grains were AFB1 and AFB2, with neither AFG1 nor AFG2 being found in any of the analyzed samples. The explanation for the detection of only group B aflatoxins is that the samples were probably contaminated only by the fungal genus A. flavus; this genus produces only B aflatoxins (Pierezan, 2013).
Samples III (1, 67µg/kg) and IV (8, 33µg/kg) showed low levels in relation to the maximum provided by national legislation. When analyzing aflatoxins in maize grains, after visual segregation of defects, researchers found that the level of contamination in grains with defects ranged from 23 to 1365 µg/kg and in grains without defects ranged from undetected to 125 µg/kg (Bento, 2016).
Contamination by aflatoxins in maize grains may have occurred in the pre-harvest, as the straw serves as a source of inoculum for the mycelium and dissemination occurs by the wind that transports the spores to the aerial part of the plant. The natural infection of A. flavus occurs mainly due to mechanical damage to the ear caused by insects whose infection begins at the tip of the ear and subsequently penetrates the embryo, causing damage or even death of the grain.
Attack by Aspergillus is commonly observed in dry and hot years and in stressed plants, mainly due to lack of nutrients and water deficiency. In the post-harvest period, the infection is mainly related to grain storage, when there is a delay in drying or even incorrect drying and storage with a moisture content above 12% (Lima, 2017).
Conclusions
In the entomological tests carried out, it was found that all the samples under study showed a strong infestation by insects, exceeding the tolerated limit for commercialization according to the current national legislation. At some points of sale (sample collection points), the maize grains from those places showed very high levels of broken, attacked, defective grains and impurities that have a direct influence on the presence of aflatoxins. Therefore, in general, based on the results of entomological tests carried out to assess the quality and safety of corn grains, it can be concluded that they do not comply with current legislation in the country, which contributes to the decrease in their commercial value.The moisture content using the technique referenced in the work, range between 13.3 and 16.5 %. Some samples from some points of sale, presented high moisture values, which may be related to the method and incomplete drying, high humidity of grains of the place of origin, storage and sale in places with fluctuations of relative humidity and proliferation of insects.It was possible detecting aflatoxin contamination using the Thin Layer Chromatography technique in three samples by AFB1 and AFB2. Regarding the quantification of aflatoxins, the AFB2
content varied from 1.67 to 8.33 µg/kg, which means they have lower levels in relation to the maximum provided by the norm of the National Institute for Standardization and Quality Control. It is in line with the HA hypothesis, which establishes that the levels of aflatoxins present in corn do not exceed the maximum acceptable limit. This means that they are suitable for human consumption but, even at low levels, aflatoxins can cause damage to health.
References
Aquino, S. L. (2003). Efeitos da radiação gama no crescimento de Aspergillus Flavus produtor aflatoxinas e no emprego da técnica da reacção em cadeia da polimerase (PCR) em amostras milho inoculadas artificialmente. Instituto de Pesquisas Energéticas e Nucleares. 11º Seminário de Ensino, Pesquisa e Extensão da Udesc Oeste – 11º SEPE & 4º Encontro da Pós-graduação da Udesc Oeste - 4º EPG. São Paulo. https://teses.usp.br/teses/disponiveis/85/85131/tde-16042012-105910/pt-br-php.
Augusto, B. (2016). Análise da Rentabilidade Económica da Produção de Milho no Distrito de Vilankulo Caso Agricultor Xibahalane (2009 a 2013). (Trabalho de Licenciatura). Escola Superior de Desenvolvimento Rural. Universidade Eduardo Mondlane. http://monografias.uem.mz/handle/123456789.
Bento, L. (2011). Qualidade física e sanitária de grãos de milho armazenados em Mato Grosso. (Tese de Mestrado). Universidade Federalde Mato Grosso, Brasil. 17-60. https://docplayer.com.br/20985224.
Bento, R. (2016). Fungos e Micotoxinas em Grãos de Milho (Zea mays l.) e seus Derivados Produzidos no Estado de Rondônia, Região Norte do Brasil. (Tese de Mestrado). Universidade Federal de Santa Catarina, Brasil. https://repositorio.ufsc.br/handle/123456789/168023.
Braga, J., Holanda, E., Barbosa, C. & Gomes, E. (2017). Detecção presuntiva de aflatoxinas em amendoins comercializados na cidade do Recife. Revista Infarma de Ciências farmacêutica, 29(2), 141-146. https://www.revistas.cff.org.br/?journal=infarma&page=article&op=vew&path%5B%5D=1839
Castro, I. M. & Anjos, M. R. (2009). Determinação de Aflatoxinas em Milho por Cromatografia Líquida de Alta Eficiência com Detecção por Fluorescência-CLAE/DF. Rio de Janeiro. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/84132/1/2009-CTE-0158.pdf.
Conceição, R. P. (2015). Qualidade Sanitária do Milho Armazenado em Propriedades Familiares Situadas na Região Central de Minas Gerais. Revista Brasileira de Agropecuária Sustentável, 4(2), 1-45. https://periodicos.ufv.br/rbass/issue/archive/3
Copetti, M. (2009). Micobiota do cacau: fungos e micotoxinas do cacau ao chocolate. (Tese de Doutoramento, Universidade Estadual de Campinas). https://core.ac.uk/download/pdf/296853616.
Domenico, A. S. (2014). Qualidade e segurança alimentar do milho em diferentes acondicionamentos de armazenagem. (Tese de Doutoramento). Universidade Estadual do Oeste do Paraná, Cascavel, Brasil. http://repositorio.utfpr.edu.br/jspui/handle/1/2149.
Drumond, V. (2012). Presença de aflatoxinas em arroz e cereais Importados na União Europeia-Revisão bibliográfica e análise de dados RASFF. (Tese de Mestrado). Universidade Nova de Lisboa, Portugal. http://repositorio.ufla.br/bitstream/1/3345/1.
Elias, M. C. & Oliveira, M. (2017). Tecnologias de Pré-Armazenamento, Armazenamento e Conservação de Grãos. Universidade Federal de Pelotas, Brasil https://bdm.ufmt.br/bitstream/1/1261/1.
Gabriel, M. (2014). Avaliação da micoflora e níveis de aflatoxinas totais em grãos de amendoim comercializados no distrito de Mocuba. (Tese de Mestrado). Departamento de Engenharia Agronómica Faculdade de Engenharia Agronómica e Florestal. Universidade Zambeze. Mocuba. https://www.researchgate.net/publication/329841212.
Heck, M.I., Defacio, R.A., Ferrer, M.E., Cirilo, A.G., Fariza, S.I., De Lucia, A.D. & Blaszchik, J.A. (2019). Evaluación de la calidad nutricional de variedades nativas de maíz de Misiones, Argentina. Revista de Investigaciones de la Facultad de Ciencias Agrarias-UNR, 34(19) 1-20. https://cienciasagronomicas.unr.edu.ar/journal/index.php/agronom/rt/printerFriendly/266/258.
Lima, A. (2017). Quantificação de fungos e de grãos avariados em milho no estado de Santa Catarina. (Tese de Mestrado). Universidade do Estado de Santa Catarina. https://www.udesc.br/arquivos/cav/id_cpmenu/1343/Disserta__o_Amanda_Lima___Quantifica__o_de_fungos_e_gr_s_avariados_em__milho_no_estado_de_Santa_Catarina_15675402724417_1343.
Mohamed, M. (2017). Factors influencing aflatoxin contamination in maize at harvest and during storage in Kongwa district, Tanzania. Sokoine University of Agriculture. Morogoro. Tanzania. https://www.suaire.sua.ac.tz/handle/123456789/2106.
Mudema, J. (2012). Rogério Francisco Sitole, et al. Rentabilidade da cultura do milho na zona sul de Moçambique: Estudo de caso do distrito de Boane.Relatório Preliminar de Pesquisa No. 3P. Instituto de Investigação Agrária de Moçambique. https://www.acismoz.com/wp-content/uploads/2017/06.
Munguambe, L. R. (2001). Síntese de Compostos Biologicamente Activos na Base de Sulfonamidas. Trabalho de Licenciatura. Departamento de Química. Faculdade de Ciências. Universidade Eduardo Mondlane. Maputo, Mozambique. http://monografias.uem.mz/jspui/handle/123456789/1279.
NM Cereais. (2009). Especificações para o milho incluindo métodos de análise e amostragem 2. https://www.portaldogoverno.gov.mz/por/content/download/4434/32874.
Oliveira, C. A. F. & Germano, P. M. (2015). Aflatoxinas: conceitos sobre mecanismos de toxicidade e seu envolvimento na etiologia do câncer hepático celular. Universidade de São Paulo, Brasil. https://www.researchgate.net/publication/26344341.
Ortega, L.O. (2023). Alimento ancestral y de subsistencia: discurso y control del cultivo de maíz en México, 1937-1961. Historia y Memoria, 27, 135-175. https://doi.org/10.19053/20275137.n27.2023.14812.
Paim, L. (2016). A fitossanidade de cereais armazenados em Angola (Tese de Mestrado). Universidade de Lisboa, Portugal. https://www.repository.utl.pt/bitstream/10400.5/12961.
Pezzini, V., Valduga, E., Cansian, R. L. (2005). Incidência de fungos e micotoxinas em grãos de milho armazenados sob diferentes condições. Revista do Instituto Adolfo Lutz 64(1), 91-96. https://periodicos.saude.sp.gov.br/RIAL/article/view/33039.
Pierezan, F. (2013). Aflatoxicose em bovinos. (Tese de Doutoramento). Universidade Federal de Santa Maria, Brasil. https://repositorio.ufsm.br/bitstream/handle/1/4077.
POCC. (2018). Plano Operacional da Comercialização de Cereais-comercialização agrícola. Ministério da Indústria e Comércio. https://www.mic.gov.mz/por/Comercio-Interno/POCA.
