Ciencia & Futuro
V. 15. No.2 junio-agosto 2025
ISSN: 2306-823X
Recibido: 18/01/2025/Aceptado: 7/05/2025
Applying Fine Coal Ash for Removing Toxic Cations in Waters of Matola River, Maputo Province
Aplicación de cenizas finas de carbón para la eliminación de cationes tóxicos en aguas del río Matola, Provincia de Maputo
Esnaider Rodríguez Suárez erodriguezsuarez2013@gmail.com (1)
Stefânia Paulo Chambal stefaniachambal96@gmail.com (1)
(1) Wutivi-UniTiva University, Boane, Maputo, Mozambique
Abstract: This study is a proposal to apply fine ashes of coal tailings to eliminate toxic cations in waters in Matola River, Maputo Province coming from industrial activities in order to enhance the material discarded after processing and minimizing environmental impacts. To fulfill the objective, ashes from fine coal tailings were synthesized by calcination method, then physicochemical and chemical characterization of the ashes and physicochemical characterization of the effluent before and after interaction with the ashes were performed by X-ray fluorescence spectrometry (XRF) and inductively coupled spectroscopy (ICP-S). After characterizing, the effluent interacted with the ashes through the adsorption process, using 0.5 g, 1 g and 2 g of ash per 50 ml of effluent, respectively. Coal ashes act as a good agent in remediating toxic cations such as Zr, Cr, As, Th, Mn, Pb and Zn present in the effluent, which validates hypothesis H1, which states that it is possible to remove toxic metal cations present in polluted waters by using fine coal ashes as adsorbent. The efficient dose for treating and removing toxic cations present in the effluent from Mozal Company is 2 g per 50 ml of effluent, since the effluent has a pH of 7.38, conductivity of 21.64 μS/cm and TDS of 10.23 mg/l.
Key words: heavy metal, metal toxicity, river pollution
Resumen: Se propone aplicar cenizas finas de relaves de carbón para eliminar cationes tóxicos en aguas del río Matola, Provincia de Maputo provenientes de actividades industriales, con el fin de valorizar el material desechado tras su procesamiento y minimizar los impactos ambientales. Para lograr el objetivo, se sintetizaron las cenizas de los relaves de carbón fino mediante el método de calcinación, luego se realizó la caracterización fisicoquímica y química de las cenizas y la caracterización fisicoquímica del efluente antes y después de la interacción con las cenizas mediante espectrometría de fluorescencia de rayos X (XRF) y espectroscopia acoplada inductivamente (ICP-S). El efluente interactuó con las cenizas a través del proceso de adsorción, utilizando 0,5 g, 1 g y 2 g de ceniza por cada 50 ml de efluente respectivamente. Las cenizas de carbón actúan como un buen agente en la remediación de cationes tóxicos como Zr, Cr, As, Th, Mn, Pb y Zn presentes en el efluente, lo que valida la hipótesis H1, que establece que es posible eliminar cationes metálicos tóxicos presentes en aguas contaminadas mediante el uso de cenizas finas de carbón como adsorbente. La dosis eficiente para el tratamiento y la eliminación de los cationes tóxicos presentes en el efluente de la empresa Mozal es de 2 g por 50 ml de efluente, ya que el mismo tiene un pH de 7,38, una conductividad de 21,64 μS/cm y un TDS de 10,23 mg/l.
Palabras clave: toxicidad por metales, contaminación fluvial, metales pesados
Introduction
Coal mining plays a historic role in the economic and sustainable development of a country, as it makes a major contribution from a socio-economic point of view due to its participation in the world energy matrix (Borba, 2001; Fuentes López, Ferrucho Parra & Martínez González, 2021).
According to José & Sampaio (2011), Mozambique has a wide range of geo-resources that are currently being exploited (Aurre & Jaén, 2015; Macamo, 2022; Wu & Dzedzemoon, 2024), such as coal in Tete province. Tete province, especially the Moatize district, has one of the largest coal deposits in the country, with reserves estimated at over 2.5 billion tons (Marove et al., 2020; Marove et al., 2022; Campos, Morais y Mafavisse, 2022). Although mineral coal is an important energy and metallurgical resource in today's society, its exploration, processing and use are potentially polluting activities, most often releasing some heavy metals that degrade the physical environment (Macie, 2015; Buitrago & Rodríguez-Aparicio, 2021; Chávez-García, 2022; González-Jiménez et al., 2023).
During coal processing, some of the material is discarded and accumulated in large areas, which is known as tailings, ranging from ROM1 (Run Of Mine) tailings to ultrafine tailings depending on their grain size (Epósito, 2000). These materials return to the mining pits causing negative effects on the environment, such as acid mine drainage, which according to Mello et al. (2012) is a phenomenon that begins when rocks containing sulphide minerals are extracted from the earth's interior by mining activities and, when disposed of on the earth's surface, oxidize by reacting with water and atmospheric oxygen.
A potential alternative for these tailings is to apply them in the treatment of liquid effluents as sorbent solids in the removal of aqueous pollutants. Among the main aqueous pollutants are toxic cations, due to their carcinogenic and mutagenic effects, which are highly harmful to living organisms.
The main aim of this research is to apply RFC ash to the removal of toxic cations in contaminated water from industrial effluent, thus helping to minimize the environmental impacts caused by the accumulation of coal tailings at the Benga - ICVL mine in the Moatize district of Tete province.
The objective of this research is to use fine coal ash for the removal of toxic cations from the polluted waters of the Matola River, Municipality of Matola, Province of Maputo.
Geographical location
The study area (Mozal company) is located in the district of Boane, in the south-east of Maputo Province, bordered to the north by the district of Moamba, to the south and east by the district of Namaacha, and to the west by the city of Matola and the district of Matutuine, and has an area of 815 km2 (MAE, 2005).
Regional geology
Geologically, the study region is part of the Mozambique Sedimentary Basin to the south of Save, covering an area of 170,000 km2. The boundary of the basin to the east is the Indian Ocean and to the west are the basaltic and rhyolitic rocks of the Karoo, which form the basis of the sedimentary deposits of the Cretaceous, whose origin is marine. In the Tertiary, formations of marine and continental origin are also known, whose deposition was controlled by marine transgressions and regressions, and in the Quaternary, eluvial and alluvial sediments.
Hydrography
The watercourses in the district of Boane belong to the Umbeluzi, Tembe and Matola river basins. The district is also crossed by the periodic Movene and Nwlate rivers (tributaries of the Umbeluzi). The most important of these is the Umbeluzi River, which rises in Eswathini and after 70 km flows into the Espírito Santo Estuary, where the Matola and Tembe rivers also have their mouths (MAE, 2005).
The Umbeluzi River is the source of drinking water for the cities of Maputo and Matola. With the growing population, the amount of water has become increasingly scarce, making it necessary to build the Pequenos Libombos Dam, as part of a strategy to use natural resources and take advantage of the region's potential (MAE, 2005).
Materials and method
The deductive method is a type of scientific method that begins with a problem or a gap in scientific knowledge, goes through the formulation of hypotheses and a process of deductive inference, which tests the prediction of the occurrence of phenomena covered by the hypothesis reference (Prodanov & Freitas, 2013).
The research in question is of an applied nature, since it seeks to apply scientific knowledge to solve collective problems which are the environmental impacts that cover society generated by the accumulation of solid industrial waste and the contamination of water by heavy metals from industrial effluents, the results of this research can be applied to solve problems that occur in reality in different parts of the country and the world, according to Gil (2019) who states that applied research covers studies designed to solve problems identified within the scope of the societies in which the researchers live. Similarly, applied research can contribute to the application of scientific knowledge and suggest issues to be investigated.
A qualitative and quantitative (mixed) research approach was used, falling within the classification of Nascimento et al., (2015), who defines it as an approach or method that employs standardized and systematic measures and also combines them with interpretation of the phenomena observed. This approach made it possible to identify and quantify the main factors influencing the incorporation of coal tailings in the remediation of waters contaminated by toxic cations.
In terms of objectives, this is an exploratory study aimed at getting closer to the problem by using the observation technique to collect samples, with the help of explanatory research.
According to Gil (2008), exploratory research provides greater familiarity with the problem. It can involve a bibliographical survey and interviews with people experienced in the problem being researched. According to the author, explanatory research aims to identify the factors that determine or contribute to the occurrence of phenomena. It is the type of research that deepens knowledge of reality, because it explains the reason, the why of things.
Technical procedures
Bibliographical research
Bibliographical research is based on materials that have already been prepared, especially books and scientific articles. Its main advantage is that it allows the researcher to cover a wide range of phenomena.
This phase consists of bibliographic research on the study of the evaluation of the potential of coal tailings, carried out on the basis of the Internet and with the help of some electronic books, scientific articles, master's theses, scientific projects, which address similar or related subjects to the coal process, solid waste management, toxic ions, methods and ways of remediating water contaminated by toxic ions and information related to the study area.
Documentary research
At this stage, we used literature acquired on the Internet and the Wutivi University library to consult works related to solid waste management. According to Gil (2008), documentary research is similar to bibliographical research, the difference being in the nature of the sources, since this form makes use of materials that have not yet received an analytical treatment, or that can still be reworked according to the research objectives.
Experimental research
The experimental activities were preceded by obtaining samples of RFC supplied by the chemistry department of Eduardo Mondlane University, and samples of effluent from industrial activities at Mozal, in Maputo province, followed by laboratory tests in order to test the hypotheses, thus implementing the thesis of Gil (2008) who states that experimental research derives from an object of study, where the variables that would be able to influence them are selected, the forms of control and observation of the effects that the variable produces on the object are defined.
Sample collection methods
The fine coal tailings from the Benga-ICVL Mine were supplied by the Chemistry Department of the Eduardo Mondlane University.
The industrial effluent sample was collected by the author at the Mozal effluent discharge point on the Matola River in Maputo province. Figure 6 illustrates the effluent sample collection process and Table 1 describes the sample collection point:
Table 1. Effluent collection point
|
Sampling points |
|
Geographical coordinates |
|||
|
|
|
Latitude |
Longest |
Elevation |
|
|
Point 1 |
25°54’40.51’’S |
32°25’10.83’’E |
6,096 meters |
||
|
Point 2 |
25°54’44.51’’S |
32°25’10.91’’E |
8,78 meters |
||
Laboratory work
This phase was carried out over a period of 45 days, in the Chemistry Department Laboratory of the Eduardo Mondlane University (UEM), preceded by a request for authorization and subsequent acceptance to carry out the laboratory tests, specifically:
a) Obtaining fine coal ash by the calcination method
b) The physical and chemical characterization of fine coal tailings ash
c) The physical and chemical composition of the effluent sample from industrial activities
d) Carrying out the adsorption processes
e) Characterization of the effluent after the intervention of the ash with the effluent
f) Consultation with environmental specialists from the chemistry department of the Faculty of Sciences at Eduardo Mondlane University
The laboratory procedures
The laboratory procedures consisted of preparing the fine coal ash and the liquid effluent and their respective physico-chemical and chemical characterizations, using the appropriate methods for each analysis, for the subsequent production of fine coal ash and finally the application of the adsorption method to remove toxic cations from the water.
Preparation of the fine coal refuse sample
The RFC sample was prepared first by grinding it in a mortar and pestle in order to reduce its particle size to a finer particle size (powder), and then weighing 200g of RFC on a technical balance.
The calcination process was carried out in order to obtain the ash from the RFC. During this procedure, 6 porcelain crucibles with a capacity of 10ml to 30ml were used, where a total of 200g of the RFC sample previously weighed on the technical balance was added, then taken to the muffle furnace with a capacity of 30-300°C, at a temperature of 850°C, for a period of 10 days, with the muffle furnace remaining on for 6 hours/day (Figure 1).
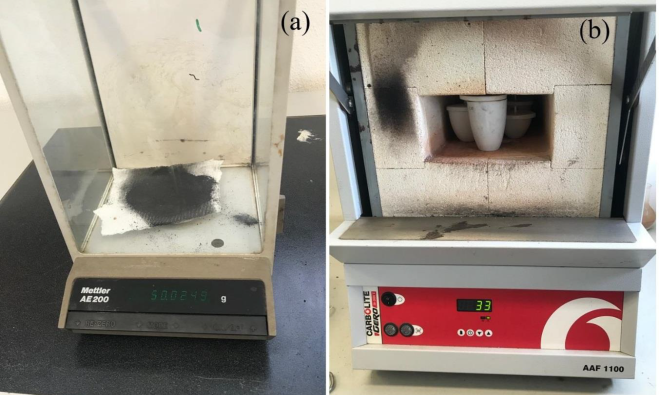
Figure 1. a) Demonstration of the weighing of the sample using the technical scale. b) calcination of charcoal in the muffle furnace using crucibles.
Preparation of the liquid effluent sample
The effluent was first prepared using filter paper. A quantity of 150 ml was filtered twice in order to retain the residues contained in the effluent that could jam the machine if the effluent did not go through the filtering process, followed by the addition of some reagents specified in Table 6, which were added to a 50ml flask for better preservation.
Sample characterization
For the physical and chemical characterization of the RFC ash and effluent samples, appropriate methods and equipment were used, each of which had its specific function for each evaluation.
Physical characterization
The physical characteristics of the samples of fine coal tailings ash and the stripe traces used were determined by visual observation and the physical characteristics of the liquid effluent before interaction with the ash and the liquid effluent after interaction with the ash were determined using materials and equipment from the laboratory of the chemistry department at UEM, where pH was analyzed using the pHmeter equipment; conductivity was determined using the conductivity meter.
Chemical characterization
The chemical characterization of coal ash was determined using XRF equipment, where the procedure consisted of placing a sample of coal ash in the sample port. Then, the time determined by the equipment's own instructions (15 min) was waited. After this time, the data obtained were entered and interpreted using a table and graph, and the chemical characterization of the effluent and influent were determined using the ICP-S method.
Discussing the results
Physical characterization
The results of the physical parameters evaluated in the ash samples were analyzed from the visual observation shown in Table 2.
Table 2. Results of analyzed physical parameters of fine coal waste ash
|
Physical Parameters Analyzed |
Results |
|
Color of Gray |
Light brown color |
|
Color of the Gray Strokes Used |
Light brown color |
The results of the table 2 reveal that the ash obtained from the fine coal waste and the ash traces used did not show any change in their initial light brownish color.
Chemical characterization
The coal ash samples analyzed using the X-ray Fluorescence technique had the analysis results illustrated in Table 3.
Table 3. Results of chemical analysis of coal ash by XRF
|
Si |
Al |
Fe |
K |
Ca |
Ti |
S |
P |
V |
Zr |
Sr |
|
46.04 |
22.52 |
14.86 |
4.57 |
4.48 |
3.74 |
1.61 |
1.15 |
0.20 |
0.18 |
0.17 |
|
Cu |
Cr |
Zn |
Rb |
Ir |
Mn |
As |
Y |
Pb |
Nb |
Th |
|
0.08 |
0.06 |
0.05 |
0.03 |
0.03 |
0.03 |
0.03 |
0.02 |
0.023 |
0.018 |
0.007 |
The result of the geochemical characterization of the ash from the fine coal waste by the XRF method demonstrates that the ash contains the major elements Si (46.04 %); Al (22.52 %) and Fe (14.86 %); medium elements such as K (4.57 %); Ca (4.48 %); Ti (3.74 %); as minor elements S (1.61 %); P (1.15 %); Cu (0.08 %); Cr (0.06 %); Zn (0.0 5%) and as trace elements V (0.20 %); Zr (0.18 %); Mr (0.17 %); Rb (0.03); Go (0.03); Mn (0.03); As (0.03); Y (0.02); Pb (0.023); Nb (0.018); Th (0.007).
Physical and chemical characterization of industrial effluent
In this phase, the results obtained in the physical and chemical characterization of the industrial effluent (before the interaction with the ash from the fine coal waste) will be presented. Table 4 presents the results of the analysis of the effluent samples using the inductively coupled plasma spectrometry (ICP-S) method.
Table 4. Results of the physical-chemical analysis of the effluent
|
Physicochemical parameters evaluated |
Concentration (mg/L) |
Maximum limit permitted by national legislation |
|
pH |
7,29 |
6.5 - 8.5 |
|
Conductivity |
22,01 |
5–20 ms/cm |
|
Total dissolved solids |
11,11 |
1000 mg/L |
|
Aluminum Content |
1.38 |
0.3mg/L |
|
Manganese content |
3.8 |
0.1mg/L |
|
Calcium Content |
79.82 |
50 mg/L |
|
Magnesium Content |
73.22 |
50mg/L |
|
Chromium Content |
0.39 |
0.05mg/L |
|
Nickel Content |
0.18 |
0.02mg/L |
|
Sodium Content |
325.33 |
200 mg/L |
|
Zinc content |
5.08 |
3.0mg/L |
|
Arsenic Content |
0.072 |
0.01mg/L |
|
Cadmium content |
0.12 |
0.03mg/L |
|
Lead Content |
0.23 |
0.1mg/L |
The results demonstrate the high content of heavy metals present in the analyzed effluent samples. Among the main toxic cations quantified, the following stand out: Aluminum with a concentration of 3.87 mg/L, Iron with 1.38 mg/L, Manganese with 3.3 mg/L, Calcium with 79.82 mg/L, Chromium with 0.39 mg/L, Sodium with 325.33 mg/L and Magnesium with 73.22 mg/L.
Physicochemical characterization of the influent after interaction with coal ash
Mushtaq et al. (2019) studied the possible applications of coal fly ash in wastewater treatment, where they illustrated that many attempts have been made by researchers for the conversion of coal fly ash into useful products while searching feasible avenues for its sustainable utilization. Wastewater remediation using coal fly ash is one such attempt solving both waste management and water quality issues. The characteristics like morphology, surface area, porosity, and chemical composition (silica, alumina, iron oxide, titania, etc.) make coal fly ash amenable material for potential application in wastewater treatment. Few reports have summarized the coal fly ash utilization in wastewater treatment but solely discussed the adsorption. Besides adsorption, the current paper aims to highlight the possibilities of using coal fly ash in wastewater treatment by different technologies that extend the utilization scope in the domains of filtration, Fenton process, photocatalysis, and coagulation. The promising use of coal fly ash as an adsorbent, membrane filter, Fenton catalyst, photocatalyst, and as an integral part of these structures is reviewed.
The physicochemical characterization of the influent (after interaction with the ash) was determined considering the hydrogen potential, electrical conductivity and total dissolved solids, and the levels of toxic heavy metals (Table 5 and Figure 2).
Table 5. Results of the physical-chemical parameters analyzed in the tributary after intermingling with ash
|
Physical-chemical parameters analyzed |
Affluent |
||
|
AM – 1 (0.5g ash) |
AM – 21 (1 g ash) |
AM – 3 (2 g ash) |
|
|
pH |
7,58 |
7,55 |
7,38 |
|
Conductivity (us/cm) |
22,14 ms/cm |
20,77ms/cm |
21,64 ms/cm |
|
TDS (mg/l) |
10,74 mg/l |
10,80 mg/l |
10,23 mg/l |
|
Aluminum |
1.03 |
0.72 |
0.14 |
|
Iron |
0.87 |
0.41 |
0.11 |
|
Manganese |
1.14 |
0.91 |
0.09 |
|
Calcium |
48.50 |
33 |
31.08 |
|
Magnesium |
63.45 |
61.08 |
48.2 |
|
Chrome |
0.12 |
0.08 |
0.031 |
|
Nickel |
0.14 |
0.09 |
0.009 |
|
Sodium |
188.95 |
132 |
112 |
|
Zinc |
3.71 |
3.04 |
2.54 |
|
Arsenic |
0.040 |
0.023 |
0.08 |
|
Cadmium |
0.09 |
0.061 |
0.024 |
|
Lead |
0.19 |
0.13 |
0.08 |
Figure 2 illustrates a comparison of the percentages of ash used to interact with the effluent samples. The results confirm the potential of fine coal waste ash as a good adsorbent of toxic cations and its remedial effect.

Figure 2. Results of the analysis of the interaction of coal ash with the effluent.
As shown in the previous graph, the dosage of 2 g of coal ash presented the best results in the efficiency of remediation of toxic cations present in the effluent. Cations such as Aluminum, which had a content of 3.87 mg/L in the effluent, after interacting with coal ash, the content dropped to 0.14 mg/L, Iron from 1.38 mg/L to 0.11 mg/L, Manganese from 3.8 mg/L to 0.9 mg/L, Calcium from 79.82 to 31 mg/L, Chromium from 0.39 mg/L to 0.031 mg/L.
Lekgoba, Ntuli y Falayi (2021), study the adsorptive properties of coal fly ash in removing copper and nickel from waste water was investigated using batch and fixed bed column studies. Various process parameters such as solution pH, solid loading, residence time, temperature, bed height and flow rate were tested to determine the effectiveness of fly ash. The study revealed that fly ash is an effective adsorbent in the selective separation of copper and nickel from binary mixtures.
The sample collected in the field (effluent) showed a pH value = 7.29, a value that is relatively low when compared to the influent samples. After mixing with 0.5g, 1g and 2g ash (designated as AM1, AM2 and AM3, respectively), the influent began to present a high pH value. This shows that tailings ash has the ability to increase the basic content of contaminated water. The results also show that the smaller the amount of ash, the more basic the influent will be.
One of the parameters that can be used as a reference for even superficial knowledge of the quality of water bodies is electrical conductivity (EC). The electrical conductivity of water represents a physical parameter used to obtain the characteristics of a given liquid medium. In terms of surface water, it is a relatively easy and quick process to characterize the medium. In general, it reflects the ability of water to conduct electrical current. This property can be a relative parameter to compare the amount of salts present. In this scientific project, of the 4 samples, the effluent shows a relatively high value, but after mixing it with the ash, there was a reduction in the conductivity value. And the greater the amount of ash in the mixture, the lower the conductivity of the influent, thus demonstrating high efficiency in controlling electrical conductivity, which in turn is related to dissolved salts.
Total dissolved solids (TDS) are directly related to electrical conductivity. In the results, after mixing the effluent with ash, the tendency of total solids is to decrease, mainly with the increase in the amount of ash. These results show the effectiveness of fine coal waste ash in reducing the total dissolved solids content in the tributary, minimizing contamination of watercourses.
Conclusions
The results of the chemical and mineralogical characterization demonstrated that the physical (macroscopic) characterization of the coal showed that it has a light brown coloration and a line of the same color.
The results of the analysis using the X-ray fluorescence method show that the major elements in the chemical composition of the ash are Si (46.04%); Al (22.52%) and Fe (14.86%); medium elements such as K (4.57%); Ca (4.48%); Ti (3.74%); as minor elements S (1.61%); P (1.15%); Cu (0.08%); Cr (0.06%); Zn (0.05%) and as trace elements V (0.20%); Zr (0.18%); Mr (0.17%); Rb (0.03); Go (0.03); Mn (0.03); As (0.03); Y (0.02); Pb (0.023); Nb (0.018); Th (0.007).
Regarding the physicochemical composition of the effluent samples, it was possible to determine the presence of toxic cations such as Zi, Cr, As, Ca, Mn, Pb, Zn among other elements using the inductively coupled spectrometry (ICP-S) method.
Regarding the physical characteristics of the effluent, it has a pH of 7.29; electrical conductivity of 22.01 μS/cm and TDS of 11.11 mg/l.
The results of the interaction of the effluent with the ash dosage of 0.5 g, 1g and 2 g demonstrated the efficiency of the ash as an adsorbent agent in the removal of toxic cations.
The efficient dosage for the treatment and removal of toxic cations present in the effluent of the Mozal company is proposed to be a dosage of 2g for 50 ml of effluent, since the effluent then had a pH of 7.38; 21.64 μS/cm of electrical conductivity and 10.23 mg/l of TDS and still manage to remediate the toxic cations present in the effluent.
Bibliographic references
Aurre, E. B., & Jaén, A. C. (2015). Extractive industries in Mozambique: threat or development opportunity? Revista CIDOB d'afers internacionals, 189-211. https://www.jstor.org/stable/43694806
Borba, R. F. (2001). Carvão mineral. Balanço mineral brasileiro, (1), 1-19. https://scholar.google.es/scholar?hl=es&as_sdt=0%2C5&q=Borba%2C+R.+F.+%282001%29.+Carv%C3%A3o+Mineral%3A+Balan%C3%A7o+mineral+brasileiro&btnG=
Buitrago, P. A. V., & Rodríguez-Aparicio, J. A. (2021). Análisis ambiental de la minería de carbón en el ecosistema estratégico de páramo (Boyacá, Colombia). Scientia et Technica, 26(03), 398-405. https://moodle2.utp.edu.co/index.php/revistaciencia/article/view/24519
Campos, V., Morais, L.C., y Mafavisse, I.M. (2022). Síntesis de un supresor de polvo de origen vegetal y pruebas en carbón de Moatize, Mozambique. Chemical and Biochemical Engineering Quarterly, 36 (3), 207-215. https://doi.org/10.15255/CABEQ.2022.2058
Chávez-García, E. (2022). Carbón mineral y biocarbón: de la revolución industrial a la captura de carbono. Contactos, Revista de Educación en Ciencias e Ingeniería, (123), 15-28. https://contactos.izt.uam.mx/index.php/contactos/article/view/179
Fuentes López, H. J., Ferrucho Parra, C. C., & Martínez González, W. A. (2021). La minería y su impacto en el desarrollo económico en Colombia. Apuntes del CENES, 40(71), 189-216. http://www.scielo.org.co/scielo.php?pid=S0120-30532021000100189&script=sci_arttext
Gil, A. C. (2008). Métodos e técnicas de pesquisa social. 6. ed. Editora Atlas SA. http://www.feata.edu.br/downloads/revistas/economiaepesquisa/v3_artigo01_globalizacao.pdf
Gil, A. C. (2019). Como elaborar projecto de pesquisa. 6ª Ed. São Paulo. Atlas. Brasil. https://files.cercomp.ufg.br/weby/up/150/o/Anexo_C1_como_elaborar_projeto_de_pesquisa_-_antonio_carlos_gil.pdf
González-Jiménez, N., Wilches-Wilches, M. R., Ramos-Parra, Y., Vargas-Rodríguez, L. J., Méndez-Arce, C., & Sandoval-Cuéllar, C. (2023). Contaminación de aire por material particulado y efectos en la salud pulmonar en población aledaña a plantas térmicas departamento de Boyacá (Colombia). Revista Investigación en Salud Universidad de Boyacá, 10(Suplemento1), 22-22. https://doi.org/10.24267/issn.2389-7325
José, D. S., & Sampaio, C. H. (2011). Estado da arte da mineração em Moçambique: Caso carvão de Moatize, Tete. Universidade Federal do Rio Grande do Sul-RS, Brasil. https://www.ufrgs.br/rede-carvao/Sess%C3%B5es_A7_A8_A9/A9_ARTIGO_03.pdf
Lekgoba, T., Ntuli, F. y Falayi, T. (2021). Aplicación de cenizas volantes de carbón para el tratamiento de aguas residuales con una mezcla binaria de cobre y níquel. Journal of Water Process Engineering, 40, 101822. https://doi.org/10.1016/j.jwpe.2020.101822
Macamo, R. D. C. (2022). Mineral resources and economic growth: evidence from the coal sector in Mozambique. Espaço e Economia. Revista brasileira de geografía económica, (24). https://journals.openedition.org/espacoeconomia/22101
Macamo, R. D. C. (2022). Mineral resources and economic growth: evidence from the coal sector in Mozambique. Espaço e Economia. Revista brasileira de geografía económica, (24). https://journals.openedition.org/espacoeconomia/22101
Marove, CA, Tangviroon, P., Tabelin, CB, e Igarashi, T. (2020). Lixiviación de elementos peligrosos del carbón y las cenizas de carbón de Mozambique. Revista Africana de Ciencias de la Tierra, 168, 103861. https://doi.org/10.1016/j.jafrearsci.2020.103861
Mello, P. A., Pereira, J. S., Mesko, M. F., Barin, J. S., & Flores, E. M. (2012). Sample preparation methods for subsequent determination of metals and non-metals in crude oil. A review. Analytica chimica acta, 746, 15-36. https://doi.org/10.1016/j.aca.2012.08.009
Ministério de Administração Estatal (MAE). (2005). Perfil do Distrito de Boane, Província de Maputo. Maputo. https://www.portaldogoverno.gov.mz/por/content/download/2965/23877/version/.../Boane.pdf
Mushtaq, F., Zahid, M., Bhatti, IA, Nasir, S. y Hussain, T. (2019). Posibles aplicaciones de las cenizas volantes de carbón en el tratamiento de aguas residuales. Revista de gestión ambiental, 240, 27-46. https://doi.org/10.1016/j.jenvman.2019.03.054
Nascimento, G. C., Dominguini, L., Mello, J. M. M., Dal Magro, J., Riella, H. G., & Fiori, M. A. (2015). Caracterização físico-química da cinza de casca de arroz oriunda do processo termelétrico do sul de Santa Catarina-Brasil. Ciência e Natura, 37(3), 634-640. https://www.redalyc.org/pdf/4675/467546194049.pdf
Wu, B., & Dzedzemoon, D. P. (2024). Evaluating the Environmental Sustainability of Silica Passivation for Acid Mine Drainage Treatment in Moatize's Coal Industry. Applied Sciences, 2(4), 13-23. https://core.ac.uk/download/pdf/613198304.pdf